RAINBOW试验是一项全球III期试验,该研究是对于转移性胃食管交界处和胃腺癌(mGC)患者给予RAM(一种人IgG1 VEGF-受体-2 靶向抗体)+紫杉醇(PTX),与安慰剂(PL)+PTX治疗方案的疗效比较分析,其证明了对患者的总生存期(OS),无进展生存期(PFS),和客观缓解率(ORR)方面有明显改善。在针对mGC的全球试验中,生存期预后的区域差异已经被报告。在该项试验中,研究人员分析了日本(JP)患者和西方(欧洲,美国,澳大利亚)患者的临床预后。下面和大家提前分享这项研究。
研究方法:
符合ECOG评分体能状态≤1,适当器官功能,以及在一线治疗方案最终剂量4个月的过程内出现疾病进展的患者纳入该项试验。采用分层log-rank检验比较OS和PFS。ORR利用Cochran-Mantel-Haenszel检验进行分析。
研究结果:
全球655例患者参与随机分配,140例是日本患者,398例是西方患者。效能结果显示于下表中。
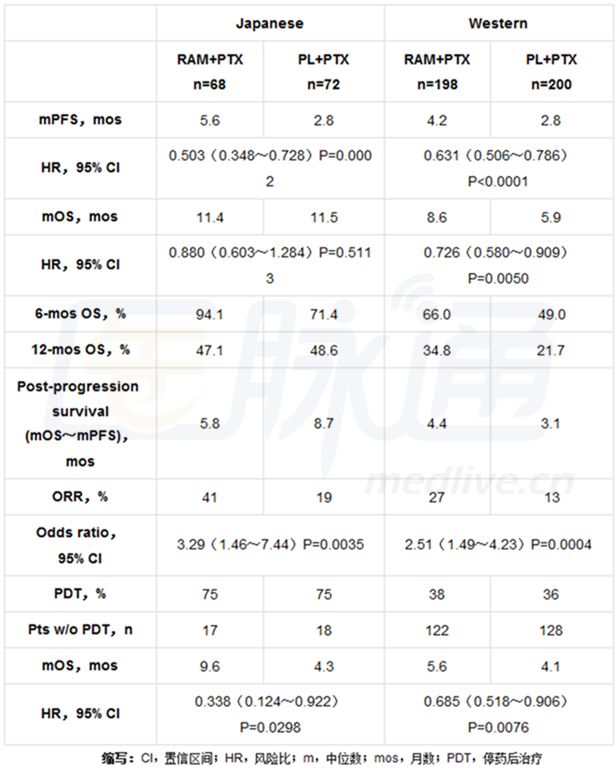
研究结论:
研究人员发现,在日本人群中,可以观察到无进展生存期,客观缓解率,以及6个月总存活率均获益,这与西方人群是一致的。日本患者进展后生存期延长可能是由于停药后处理(PDT)的较高使用,或许掩盖了潜在的总生存期获益。
医脉通整理报道,转载请注明出处。
会议专题》》》2014年ASCO年会专题报道
阅读摘要原文
RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab (RAM) plus paclitaxel (PTX) versus placebo (PL) plus PTX in the treatment of metastatic gastroesophageal junction and gastric adenocarcinoma (mGC) following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy—Efficacy analysis in Japanese and Western patients.(Abstract4005)
Authors: Shuichi Hironaka, Yasuhiro Shimada, Naotoshi Sugimoto, et al.
Session Type: General Poster Session
Background: RAINBOW, a global phase III trial, demonstrated significant improvements in overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) in patients (pts) with mGC receiving RAM, a human IgG1 VEGF-receptor-2 targeted antibody, plus PTX compared with PL plus PTX. In global trials for mGC, regional differences in survival outcomes have been reported. Here, we analyzed clinical outcomes of Japanese (JP) pts and Western (Europe, US, Australia) pts.
Methods: Eligibility included Eastern Cooperative Oncology Group performance status ≤1, adequate organ function, and disease progression during or within 4 months of the last dose of first-line therapy. OS and PFS were compared using a stratified log-rank test. ORR was analyzed using a Cochran-Mantel-Haenszel test.
Results: Of 665 patients randomized worldwide, 140 were JP pts and 398 were Western pts. Efficacy results are shown in the Table.
Conclusions: Benefit was seen in PFS, ORR, and the 6-mos OS rate in the JP population, which was consistent with the Western population. Prolonged post-progression survival in JP pts may be due to higher use of post-discontinuation treatment (PDT) and may have masked the potential OS benefit. Clinical trial information: NCT01170663.
