甲磺酸艾日布林(eribulin)是一种新型的非紫杉烷类细胞微管动力抑制剂,是一种软海绵素类的抗肿瘤药物,用于以前应用过两种或两种以上的化疗方案的转移性乳腺癌患者。以前的一个关于应用艾日布林+曲妥单抗一线治疗HER2+乳腺癌患者的II期试验显示,客观缓解率为71%,耐受性与已知的这类药物的耐受性一致。美国临床肿瘤学学会主办的乳腺癌研讨会上公布的一项研究评价了艾日布林+曲妥单抗一线治疗HER2阳性转移性乳腺癌的疗效。
研究方法
以前未应用过化疗的HER2+转移性乳腺癌患者在第1天和第8天静脉注射甲磺酸艾日布林1.4 mg/m2,首个疗程第1天也静脉注射曲妥单抗8 mg/kg,随后的每个疗程中应用6 mg/kg。评估以前应用或未应用过曲妥单抗的患者的缓解率、无进展生存期和耐受性。
研究结果
52位患者(年龄的中位数为59.5年)应用艾日布林+曲妥单抗联合治疗,治疗的时间的中位数为30周;40% (n=21)的患者以前应用过曲妥单抗进行新辅助/辅助治疗。上次完成辅助治疗到应用艾日布林+曲妥单抗一线治疗转移性乳腺癌的时间的中位数为23个月。应用客观缓解率、临床获益率、无进展生存期和缓解的持续时间评估疗效。以前应用过曲妥单抗的患者和以前未使用过的患者的很大程度上是一致的(见表)。各组间3-5级的不良反应、治疗相关的不良反应和治疗中断率是相似的(表)。
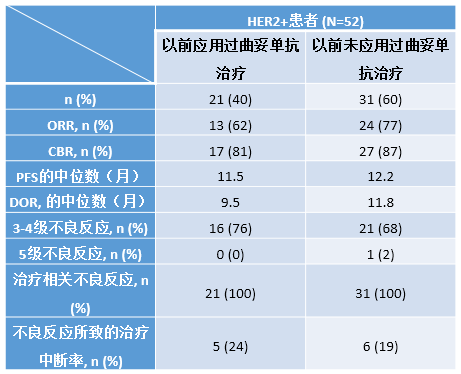
表.ORR:客观缓解率;CBR:临床获益率;PFS:无进展生存期;DOR:缓解的持续时间
结论
在HER2+的转移性乳腺癌患者中进行的这一II期单组试验中,不管以前是否应用过曲妥单抗进行(新)辅助治疗,艾日布林+曲妥单抗作为一线治疗的疗效都很好,耐受性也很好。临床试验信息:NCT01269346。
阅读原文摘要
Clinical effects of prior trastuzumab on combination eribulin mesylate plus trastuzumab as first-line treatment for HER2+ locally recurrent or metastatic breast cancer (MBC): Results from a phase 2, single-arm, multicenter study.
Background
Eribulin mesylate, a novel nontaxane microtubule dynamics inhibitor in the halichondrin class of antineoplastic drugs, is indicated for women with MBC who previously received ≥ 2 chemotherapy regimens in the metastatic setting. Primary data from a phase 2 trial on first-line combination eribulin + trastuzumab (TRAS) in HER2+ patients (pts) showed a 71% objective response rate (ORR) and tolerability consistent with the known profile of these agents. Here we present prespecified endpoint data for this study by prior TRAS use.
Methods
Pts with HER2+ MBC who had not received prior chemotherapy for MBC received eribulin mesylate 1.4 mg/m2 IV on days 1 and 8 of each-day cycle and initial TRAS (8 mg/kg IV/day 1), followed by 6 mg/kg/day 1 of each subsequent cycle. Response, progression-free survival (PFS), and tolerability were assessed in patients who had and had not received prior TRAS treatment.
Results
The 52 pts (median age, 59.5 years) received combination eribulin + TRAS, for a median treatment duration of ~30 weeks; 40% (n=21) were previously treated with TRAS in the neo-adjuvant/adjuvant setting. There was median of 23 months since completion of adjuvant treatment prior to retreatment with eribulin + TRAS for first-line MBC. Efficacy, assessed by ORR, clinical benefit rate (CBR), PFS, and duration of response (DOR), was largely consistent in pts who received prior TRAS relative to pts who had not received prior TRAS (see table). Overall, grade (G) 3-5 adverse events (AEs), treatment-related AEs (TRAEs), and discontinuations (d/c) were similar between groups (Table).
Conclusions
In this phase 2 single-arm trial in pts with HER2+ MBC, eribulin + TRAS demonstrated activity and was well tolerated as first-line treatment, irrespective of prior (neo) adjuvant TRAS treatment. Clinical trial information: NCT01269346.
