Veliparib(V)(ABT-888)是一种口服、强效PARP 1/2抑制剂。在临床前期和临床试验中,PARP抑制剂对BRCA+恶性肿瘤有效。BRCA+癌症、浆液性卵巢癌和基底细胞样乳腺癌的基因型和表型具有相似性。因此,研究人员推断这些肿瘤类型对PARP抑制剂单药治疗的敏感性相似。
2014年美国临床肿瘤学学会主办的乳腺癌研讨会上的一项试验,为确定两组患者(BRCA+和BRCA野生型患者,包括浆液性卵巢癌和三阴性乳腺癌)长期服用Veliparib的最大耐受剂量(MTD)、剂量限制毒性(DLT)、药代动力学和药效动力学特点以及初步疗效展开了研究。
研究方法:
研究人员进行了一项3+3的剂量递增I期试验。计划应用9种剂量层次,并从50mg(一天两次)开始剂量递增至最大剂量500mg(一天两次),从而确定最大耐受剂量和II期试验的推荐剂量。持续口服Veliparib,每28天一个疗程。BRCA+和BRCA野生型患者被纳入两个不同的研究队列中(分别进行剂量递增试验)。
研究结果:
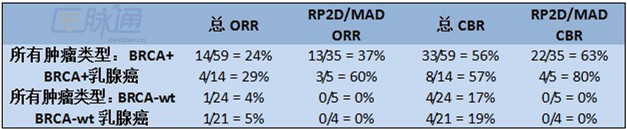
一共有98例患者纳入该项研究,其中BRCA+的患者有70位,BRCA野生型的患者有28位。两组患者最大的注射剂量为500mg(一天两次),最大耐受剂量/II期试验的推荐剂量为400mg(一天两次)。59位BRCA+患者和24位BRCA野生型患者(21位三阴性乳腺癌和3位卵巢癌患者)可用于缓解评估。ORR定义为CR+PR和临床缓解率(CBR),即CR+PR+SD>6个月。结果见表格。
研究结论:
有证据表明,在BRCA+的患者中,联合Veliparib抗肿瘤活性与它PARP抑制剂相媲美。有迹象表明在这类患者人群中,随着剂量的递增疗效也逐渐提高。在大多数三阴性乳腺癌、BRCA野生型乳腺癌患者中疗效比较差,尽管有证据表明其在一小部分患者中有效。正在进行的相关试验可能会找出敏感性和耐药性的潜在机制。临床试验信息:NCT00892736。
阅读研究原文:
Outcome of BRCA 1/2-Mutated(BRCA+)and triple-negative, BRCA wild type(BRCA-wt)breast cancer patients in a phase I study of single-agent veliparib(V)
Background: Veliparib (V) (ABT-888) is an oral, potent inhibitor of PARP 1/2. PARP inhibitors have preclinical and clinical efficacy in BRCA+ malignancies. There are genotypic and phenotypic similarities between BRCA+ cancers, serous ovarian cancer and basal-like breast cancer and we postulated that these tumors types may be similarly sensitive to single-agent PARP inhibition. This study sought to establish the maximum tolerated dose (MTD), dose -limiting toxicities (DLT), pharmacokinetic and pharmocodynamic properties, and preliminary efficacy of chronically-dosed V in 2 cohorts of patients, BRCA+ and BRCA-wt (consisting of serous ovarian cancer and triple-negative breast cancer (TNBC).
Methods: A 3+3 dose escalation phase I trial was performed. Nine dose levels(DL)were planned, and dose escalation started at 50 mg BID to a maximum of 500 mg BID to determine a maximum tolerated dose(MTD)and recommended phase II dose(RP2D). V was administered orally continuously on a 28 day cycle. BRCA+ and BRCA-wt patients were enrolled in 2 separate cohorts with 2 separate escalations.
Results: 98(70 BRCA+ and 28 BRCA-wt)pts have been enrolled. The maximum administered dose(MAD)was 500mg BID and the MTD/RP2D is 400mg BID for both cohorts. 59 BRCA+ pts and 24 BRCA-wt pts(21 TNBC and 3 ovary)were evaluable for response. ORR was defined as CR+PR and clinical benefit rate(CBR)as CR+PR+SD>6 months. Results are summarized in the table.

Conclusions: There is evidence of anti-tumor activity with V comparable to that of other PARP inhibitors in the BRCA+ population. There was indication of dose responsiveness with greater activity in this population at higher doses. There is less activity in the mostly TNBC, BRCA-wt population, although there was evidence of benefit in a small number of patients. Ongoing tissue correlative studies will help to identify potential mechanisms of sensitivity and resistance. Clinical trial information: NCT00892736.
