第51届美国临床肿瘤学会(ASCO)年会将于5月29日至6月2日在芝加哥召开。由中山大学第六医院的邓艳红教授、汪建平教授担任主要研究者的中国研究——FOWARC研究,入选了2015年ASCO口头报告(摘要号,3500)。该研究是唯一入选2015ASCO年会口头报告的中国研究。该研究的初步结果将在当地时间5月30日下午召开的结直肠癌专场上公布。医脉通带大家先睹为快。
FOWARC研究调查了术前mFOLFOX6化疗(CT)是否可以改善局部晚期直肠癌患者的无病生存(DFS)。
在2011年1月至2015年2月期间,临床II-III期的、距肛门边缘12cm内的直肠癌患者被随机分配接受5-FU联合放疗(对照组),或接受mFOLFOX6联合放疗(FOLFOX-放疗组),或接受4到6个周期的单独mFOLFOX6治疗(FOLFOX组),如果需要,可以允许采用术后放疗。研究的首要终点为DFS。ASCO会议上将公布的该研究的初步结果如下。
研究共纳入了495位患者(每组165位患者)。FOLFOX-放疗组中有92%的患者完成了至少46Gy的放疗,而对照组完成的有86.8%。5%的FOLFOX组患者接受了术后放疗。在对照组,FOLFOX-放疗组及FOLFOX组中的RO切除率分别为90.1%, 88.2%, 及91.2%。三组的pCR率分别为12.5%, 31.3% 及7.4% (P = 0.001)。三组分期下调(达到ypTNM 0-1)率分别为34.7%, 57.8%,及37.9%。接受放疗的患者中观察到了更大的毒性和术后并发症。病灶距肛缘5cm内的患者亚组也有相似的结果。
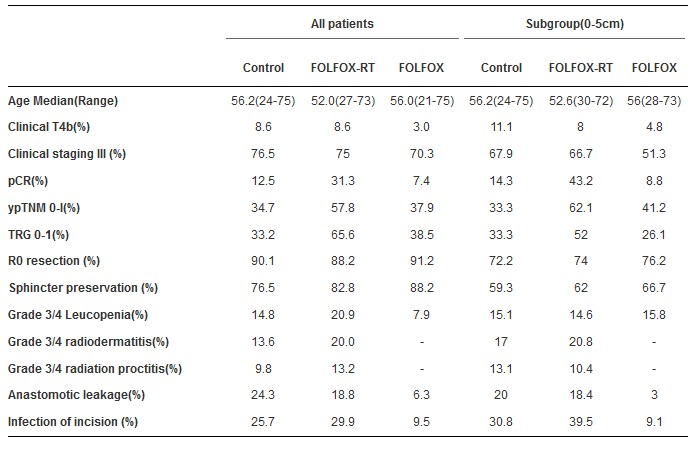
研究发现,mFOLFOX6与放疗同步可达到高的pCR率。与术前5-FU 联合放疗相比,单独mFOLFOX6新辅助治疗可以得到相似的分期下降(达到ypTNM 0-1)结果,并且毒性更小,术后并发症更少。临床信息:NCT01211210
摘要原文:
Background: The FOWARC study investigates whether peri-operative mFOLFOX6 chemotherapy (CT) improves disease-free survival (DFS) in locally advanced rectal cancer.
Methods: Between 01/2011-02/2015, patients with rectal cancer within 12 cm from the anal verge, clinical stage II-III were randomly assigned to received 5-FU with radiation(RT) (control arm), or receive mFOLFOX6 with RT (FOLFOX-RT arm), or receive 4-6 cycles of mFOLFOX6 alone (FOLFOX arm), post-operative RT is allowed if needed. Primary endpoint is DFS. Here we report the preliminary results.
Results: 495 patients were randomized (165 in each arm). 92% of patients accomplished at least 46 Gy of RT in FOLFOX-RT arm compared to 86.8% in control arm. 5% of FOLFOX arm patients received post-op RT. R0 resection rate was 90.1%, 88.2%, and 91.2%, respectively in control arm, FOLFOX-RT arm and FOLFOX arm. The pCR rate was 12.5%, 31.3% and 7.4% respectively (P = 0.001). Good down staging (ypTNM 0-1) was achieved in 34.7%, 57.8%, and 37.9% of patients respectively. Higher toxicity and post-op complications were observed in patients received RT. Similar results were seen in subgroup of patients with lesions located within 5cm from the anal verge.
Conclusions: mFOLFOX6 concurrent with RT resulted in higher pCR rate, neoadjuvant mFOLFOX6 alone achieved similar down staging rate with less toxicity and post-op complications, compared to preoperative 5-FU with RT. Clinical trial information: NCT01211210
更多中国专家的壁报研究,敬请期待……
