医脉通编译,转载请务必注明出处
2015年ASCO年会于5月29日—6月2日在美国芝加哥召开。5月31日上午的消化系统(非结直肠)肿瘤口头报告专场上,一项III期,随机,双盲,多中心,对照试验RILOMET-1研究,在晚期MET-阳性胃或胃食管交界处(G/GEJ)肿瘤患者中,将rilotumumab(R)联合表柔比星、顺铂和卡培他滨(ECX)作为一线方案治疗与安慰剂(P)进行比较。医脉通整理如下:
Rilotumumab(R)是一种肝细胞生长因子的完全人源化单克隆抗体。R+ECX vs P+ECX治疗MET-阳性G/GEJ肿瘤的II期临床试验显示R+ECX能够提高总生存期(OS)和无进展生存期(PFS)(参考文献:Lancet Oncol 2014;15:1007)。该项III期临床试验评估了R+ECX治疗MET-阳性G/GEJ肿瘤的疗效和安全性。
在研究中主要入组标准是,年龄≥18年;既往未经治疗,病理证实未不可切除的晚期G/GEJ腺癌;ECOG评分0-1分;肿瘤免疫组化MET阳性;HER2阴性。患者按1:1随机接受ECX(静脉表柔比星50mg/m2 D1,静脉顺铂60mg/m2 D1,口服卡培他滨625mg/m2 BID D1-21)联合R 15mg/kg或安慰剂(P)静脉Q3W治疗,对疾病侵及范围(局部晚期 vs 转移性)和ECOG评分(0 vs 1)进行分层。主要终点为OS。随机因素分层后,采用对数秩检验比较组间OS。本研究预计HR为0.69。关键次要终点是PFS,12个月存活率,客观缓解率(ORR),安全性和药代动力学(PK)。
在2012年11月至2014年11月,随机入组609例患者。该项研究因组间死亡病例失衡而提前终止(死亡病例R vs P:128 vs 107,截止数据:2014年11月27日)。R组OS并不优于P(组单侧检验,P值=0.99)。R组统计的OS,PFS和ORR更差。而且R组中所有的亚组均未见受益,包括MET表达≥1+的高表达肿瘤细胞人群。R组常见副反应(外周水肿,低蛋白血症,深静脉血栓和低钙血症)的发生率更高。
因此,RILOMET-1试验未达到研究主要终点;R组的OS更差,且差异具有统计意义。本研究的药代动力学和MET生物标志物分析仍在进行中。临床试验信息:NCT01697072。
会议专题》》》2015年ASCO年会专题报道
阅读原文摘要
Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study.(Abstract 4000)
Authors:David Cunningham, Niall C. Tebbutt,et al.
Session Type:Oral Abstract Session
Background:R is a fully human monoclonal antibody to hepatocyte growth factor. A phase 2 study showed improved overall survival (OS) and progression-free survival (PFS) with R + ECX vs P + ECX in MET-pos G/GEJ cancer (Lancet Oncol 2014;15:1007). This phase 3 trial evaluated the efficacy and safety of R + ECX in MET-pos G/GEJ cancer.
Methods:Key eligibility criteria: ≥ 18 yr; previously untreated, pathologically confirmed unresectable advanced G/GEJ adenocarcinoma; ECOG score 0–1; tumor MET-pos by IHC; HER2-negative. Pts were randomized 1:1 to receive ECX (IV epirubicin 50 mg/m2 D1, IV cisplatin 60 mg/m2 D1, oral capecitabine 625 mg/m2BID D1−21) + R 15 mg/kg or P IV Q3W and stratified by disease extent (locally advanced vs metastatic) and ECOG score (0 vs 1). Primary endpoint: OS. A log-rank test stratified by randomization factors compared OS between arms. The study was powered to detect a HR of 0.69. Key secondary endpoints: PFS, 12-mo survival rate, objective response rate (ORR), safety and pharmacokinetics (PK).
Results:609 pts were randomized from Nov 2012 to Nov 2014. The study was stopped early based on an imbalance in deaths (R vs P: 128 vs 107 deaths, data cutoff: 27 Nov 2014). R was not superior to P for OS (one-sided test, p = 0.99). OS, PFS and ORR were statistically worse in the R arm. No subgroups seemed to benefit with R, including those with higher percentages of cells with ≥ 1+ MET expression. Most common AEs that were higher with R: peripheral edema, hypoalbuminemia, deep vein thrombosis and hypocalcemia.
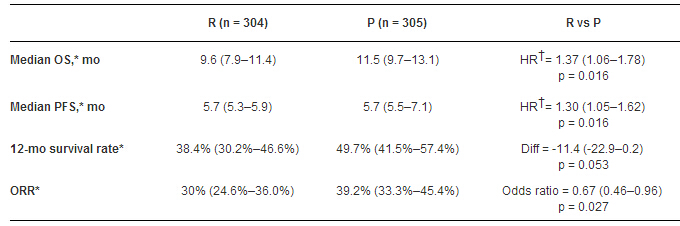
Conclusions:RILOMET-1 did not meet its primary endpoint; OS was statistically significantly worse with R. PK and MET biomarker analyses are pending. Clinical trial information: NCT01697072
