医脉通编译,转载请务必注明出处
2015年ASCO年会于5月29日—6月2日在美国芝加哥召开。5月31日上午的消化系统(非结直肠)肿瘤口头报告专场上,公布了一项摘要号为4003的INTEGRATE试验结果,在难治性晚期胃食管癌(AOGC)患者中,开展了一项瑞格菲尼(regorafenib)治疗的随机、II期、双盲、安慰剂对照研究。医脉通整理如下:
瑞格菲尼(REG)是一种口服多激酶抑制剂,需要在一线和二线化疗失败的难治性晚期胃食管癌患者中评估其疗效,因为这些患者在化疗失败后几乎无可选治疗方案。
该项研究是一国际化(澳大利亚、新西兰(ANZ)、韩国、加拿大(NCIC CTG))II期随机对照试验,患者以2:1随机入组到160mg REG组和安慰剂(PBO)组,给药方案d1-21,每28天重复,直至疾病进展(PD)或不良反应无法耐受。主要终点:无进展生存期(PFS)。最终分析中纳入的数据截至2014年12月31日。
入组研究的患者有152例(2012年11月至2014年2月),其中可评估的患者147例(REG组:97例和PBO组:50例)。男:女(118:29);原发部位:胃食管交界(56),胃(85);既往化疗:一线(62),二线(85);ECOG PS评分:0(62),1(85)。中位治疗周数:8(REG)vs 4(PBO)。中位REG剂量强度:150mg(130mg韩国和160mg ANZ/Can)。PBO组中有27例患者在疾病进展后接受了REG治疗。中位PFS,REG组11.1周(95%CI:7.7-13.3),而PBO组3.9周(3.7-4.0),HR是0.40,P<0.0001。中位OS,REG组25周(95%CI:18.9-29.6)vs PBO组19.4周(95%CI:14.9-22.7),HR是0.74,P=0.11。
预先指定分析发现韩国REG的疗效明显优于ANZ/Can(HR 0.12 vs 0.61,P=0.0009),但与年龄、NLR、原发部位、化疗阶段、存在腹膜转移、转移病灶数量、VEGF-A(表)等因素一致。本项研究中,REG耐受性良好,且毒副反应在预期的范围内。
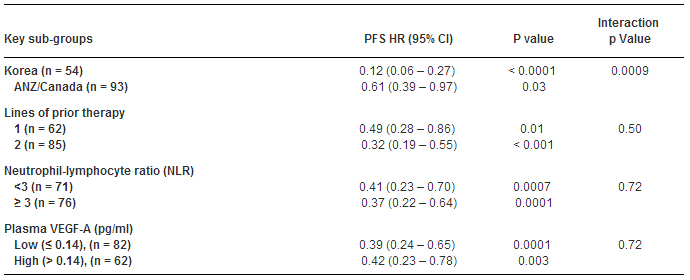
因此,研究人员认为对大部分患者,REG能够有效地延长的PFS,但在OS上无显著优势。REG的疗效具有地区差异,但REG在所有地区及亚组中均是有效的。进一步研究结果有待于III期试验。临床试验信息:12612000239864。
会议专题》》》2015年ASCO年会专题报道
阅读原文摘要
INTEGRATE: A randomized, phase II, double-blind, placebo-controlled study of regorafenib in refractory advanced oesophagogastric cancer (AOGC): A study by the Australasian Gastrointestinal Trials Group (AGITG)—Final overall and subgroup results.(Abstract 4003)
Authors:Nick Pavlakis, Katrin Marie Sjoquist,et al.
Session Type:Oral Abstract Session
Background:REG is an oral multi-kinase inhibitor warranting evaluation in AOGC following failure of 1st or 2nd line chemotherapy (CT) where few options exist.
Methods:International (Australia & New Zealand (ANZ), Korea, Canada (NCIC CTG)) phase II RCT with 2:1 randomization to 160 mg REG or matched Placebo (PBO) on days (D) 1-21 each 28 D cycle until disease progression (PD) or prohibitive adverse events. Primary endpoint: progression free survival (PFS). Final analysis used data to December 31, 2014.
Results:152 patients (pts) enrolled (November 2012 to February 2014) yielding 147 pts evaluable for analysis (97 REG and 50 PBO). M:F (118:29); primary site: OGJ (56), stomach (85); lines of prior CT: 1 (62), 2 (85); ECOG PS 0 (62): 1 (85). Median (med.) treatment wks: 8 (REG) v 4 (PBO). Med. REG dose intensity: 150 mg (130 mg Korea and 160mg ANZ/Can). 27 PBO pts received REG following PD. REG Med. PFS 11.1 wks (95% CI: 7.7 – 13.3) v PBO 3.9 wks (3.7 - 4.0), HR 0.40, p < 0.0001. Med. REG OS 25 wks (95% CI: 18.9-29.6) v PBO 19.4 wks (95% CI: 14.9 – 22.7), HR 0.74, p = 0.11. Pre-specified analyses found REG effect greater in Korea than ANZ/Can (HR 0.12 v 0.61, p = 0.0009) but consistent across age, NLR, primary site, lines of CT, peritoneal metastases (mets) presence, number of met. sites, and VEGF-A (Table). Results comparable for ITT population (n = 152). REG was well tolerated, with expected spectrum of toxicities.
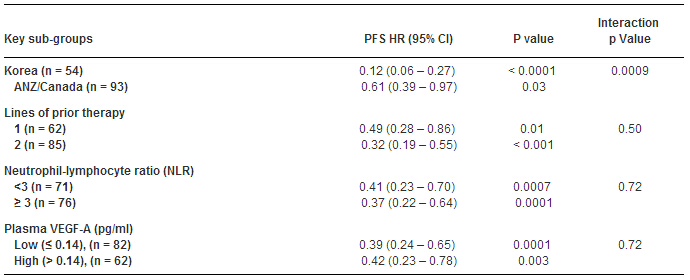
Conclusions:REG was highly effective in prolonging PFS across a broad range of pts, with a non-significant positive OS trend. Regional differences were found in the magnitude of effect but REG was effective in all regions and subgroups. A phase III trial is merited. Clinical trial information: 12612000239864.
