医脉通编译整理,转载请务必注明出处。
2016 ESMO大会上,免疫治疗是肺癌领域的重头戏,除了PD-1抑制剂Pembrolizumab在PD-L1高表达患者中成功跃居晚期非小细胞肺癌(NSCLC)一线治疗梯队外,作为配体的PD-L1抑制剂Atezolizumab的Ⅲ期临床研究OAK也取得阳性结果。与化疗相比,Atezolizumab可显著改善经治NSCLC患者的生存。
PD-L1抑制剂(注意不是PD-1)是从配体的角度,阻断PD-1与PD-L1的结合,阻断负向调控信号,使T细胞恢复活性,从而增强抗肿瘤免疫应答。
主要研究者,来自法国艾克斯-马赛大学的Fabrice Barlesi博士说:“肿瘤免疫治疗的目的是使免疫系统能控制甚至消灭癌细胞,因此Atezolizumab可能在非常多的肿瘤中发挥作用。”
OAK研究纳入1225例接受过其他治疗的NSCLC患者,根据PD-L1状态、先前化疗方案数量以及组织学将其分层,之后随机进入Atezolizumab治疗(1200mg,iv)或多西他赛(75mg/m2)治疗。
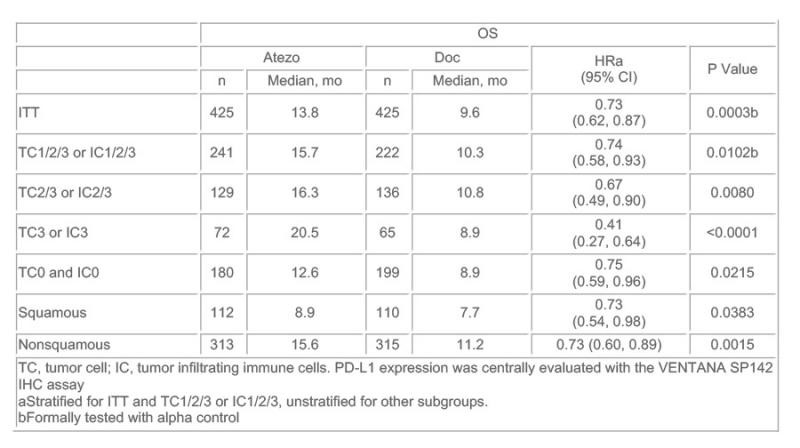
850例患者数据的初步分析显示,不考虑PD-L1表达(包括<1%)情况,Atezolizumab治疗与多西他赛相比使患者的总生存(OS)提高27%。
当根据PD-L1表达水平将患者分层后,PD-L1表达最高的患者队列中Atezolizumab治疗与多西他赛相比,可使患者的总生存(OS)提高59%,然而,即使是在PD-L1无表达的患者中,Atezolizumab仍然可使OS显著改善25%。组织类型方面,腺癌和肺腺癌患者的OS获益接近。
Barlesi博士说:“这是PD-L1抑制剂Atezolizumab的首个Ⅲ期临床研究,该研究验证了POPLAR Ⅱ期研究中该药显现出的疗效,且与PD-1抑制剂治疗结果相符。Azetizolumab为晚期NSCLC患者提供了另一种新的二线治疗选择,而且可以不用考虑肿瘤的PD-L1表达情况。”
在Ⅱ期PORLAR临床试验中,ITT人群的OS在多西他赛化疗组为9.7个月,而Atezolizumab组为12.6个月,提升明显(HR=0.73)。
德国Grosshansdorf肺科诊所教授Martin Reck在评论该研究时表示:“这为PD-1/PD-L1在NSCLC治疗中的地位提供了重要信息,证实了POPLAR研究和CHECKMATE系列研究的生存获益。有趣的是,OAK研究中无PD-L1表达的患者也有OS获益,意味着用PD-L1阴性排除患者的标准存在问题。”
在描述治疗活性时,PD-L1可能是一种很好的补充,但并不是一个理想的替代标志物。未来还需要其他标志物来判断患者从免疫治疗中的获益与否。
信源:Significant Survival Gains with Atezolizumab vs Docetaxel for Non-Small-Cell Lung Cancer. ESMO 2016 Press Release.
摘要原文
Abstract:LBA44
Title:Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC
Background: Atezolizumab (atezo) inhibits the binding of PD-L1 to its receptors PD-1 and B7.1, thereby restoring tumor-specific T-cell immunity, while leaving the PD-L2/PD-1 interaction intact. Atezo demonstrated survival benefit vs docetaxel (doc) in the Ph2 trial POPLAR. Here we present the primary analysis from the Ph3 OAK study evaluating atezo vs doc in previously treated NSCLC.
Methods: Previously treated NSCLC patients (pts) were stratified by PD-L1 status, prior chemotherapy regimens (1 vs 2) and histology, and randomized 1:1 to atezo (1200 mg IV q3w) or doc (75 mg/m2 IV q3w). The co-primary endpoints were OS in the ITT and PD-L1–expression subgroup TC1/2/3 or IC1/2/3 (PD-L1 expression on ≥ 1% TC or IC). Secondary endpoints included PFS, ORR, DoR and safety.
Results: The primary efficacy analysis was conducted in the first 850 of 1225 total enrolled pts. Pts had a median age of 64 y, 61% were male, 25% had 2 prior lines of therapies, 26% had squamous histology, 67% were previous smokers and 37% were PS 0. Superior OS was seen with atezo vs doc in ITT (HR 0.73; P = .0003) and TC1/2/3 or IC1/2/3 pts (HR 0.74; P = .0102). Survival was improved regardless of PD-L1 expression levels, including in pts with no PD-L1 expression (TC0 and IC0). There was pronounced benefit in pts with high PD-L1 expression (TC3 or IC3). OS benefit was similar in pts with squamous or nonsquamous histology. In ITT pts, PFS HR was 0.95 (2.8 vs 4.0 mo), ORR 13.6% vs 13.4%, and DoR 16.3 vs 6.2 mo for atezo vs doc. Gr 3-4 treatment-related AEs occurred in 15% of atezo pts and 43% of doc pts. There were no deaths related to atezo and 1 related to doc. No new safety signal was observed.
Conclusions: This first Ph3 trial of a PD-L1-directed drug in NSCLC demonstrates that atezo treatment results in a statistically significant and clinically relevant improvement in OS vs doc in 2L/3L NSCLC, regardless of PD-L1 expression and histology. Atezo was well tolerated with a favorable safety profile vs doc.