2016年12月4日-7日,第17届世界肺癌大会(WCLC)在奥地利维也纳盛大召开。会议进行到第4天(当地时间7日的上午),公布了4项重要免疫治疗研究成果。
来自意大利IRCCS医学中心的Marina Garassino教授报告了ATLANTICⅡ期研究结果。报告内容涉及Durvalumab用于经多线治疗后局部晚期或转移性NSCLC的治疗效果,以及PD-L1表达对Durvalumab治疗效果的影响。
研究背景
业已证实,抗PD-1/PD-L1抗体免疫治疗晚期NSCLC可以为患者带来显著临床获益。但对于二线化疗失败疾病进展的患者,治疗选择少,预后差。Durvalumab是一种人源IgG1单克隆抗体,靶向细胞程序性死亡因子配体1(PD-L1)。
研究内容
ATLANTIC(NCT02087423)是一项开放标签的Ⅱ期单臂临床试验,治疗对象为局部晚期或转移性ⅢB~IV期NSCLC患者,要求WHO PS 0或1,继往接受2种以上系统性化疗(包括含铂化疗)。
研究未对曾经的治疗方案数量设定上限。初期所纳入的患者较为松散,而后期限制为PD-L1高表达(≥25%的肿瘤细胞膜染色)的患者。
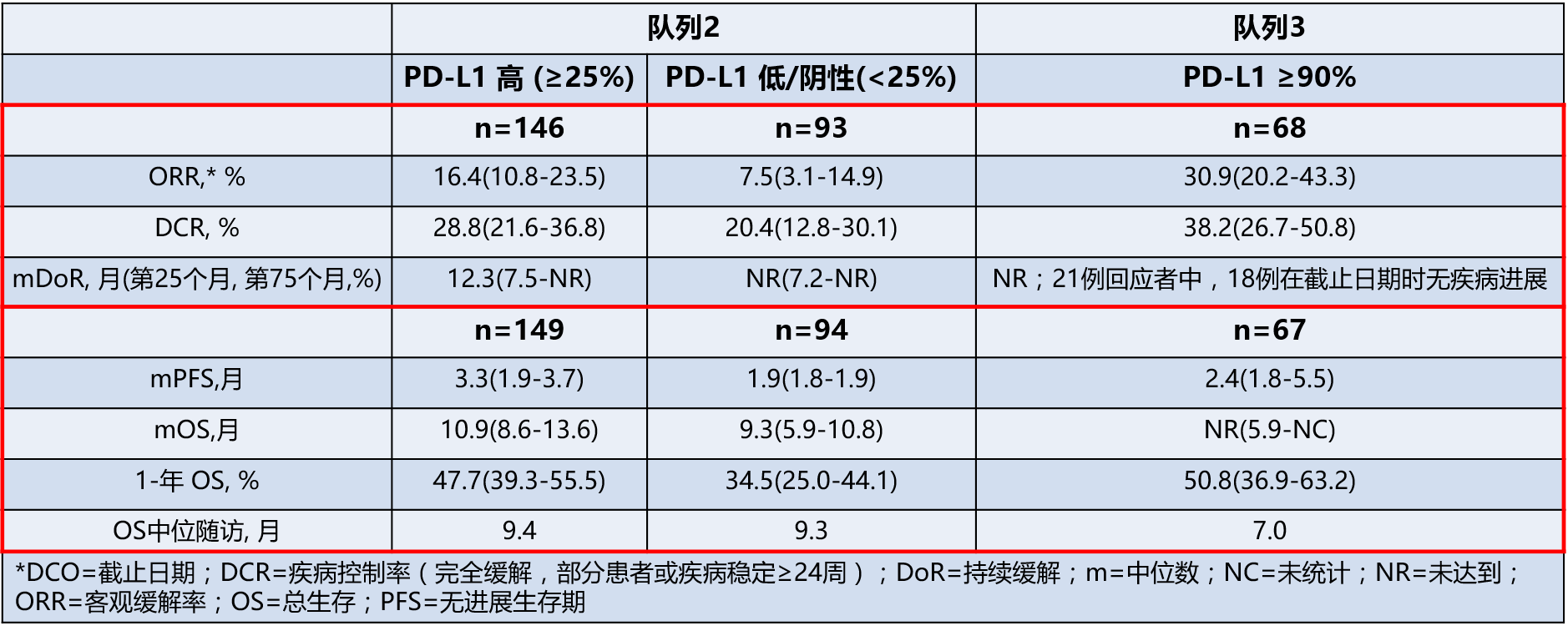
该研究包含3个患者队列。2016WCLC上研究者报告了EGFR/ALK野生型或突变状态未知的两个患者队列的研究结果。队列3中,90%以上的肿瘤细胞PD-L1染色阳性。
主要终点为客观缓解率(ORR);次要终点包括疾病控制率(DCR),持续缓解(DoR),无进展生存期(PFS),总生存(OS)和安全性。
主要结果
截止到2016年6月3日,在队列2和队列3中,分别有265例和68例患者给予Durvalumab注射治疗(10 mg/kg,每2周1次)。
◆队列2:受试者265例,中位年龄62岁,PS 1 67%,鳞癌占21%;经治平均次数为3.2次。
◆队列3:受试者68例,中位年龄61岁,PS 1 72%,鳞癌占29%;经治平均次数为2.6次。
在队列2中,对于PD-L1≥25%的患者鳞癌或非鳞癌ORR相近。
低级别的不良事件最常见,可通过延迟治疗和(或)免疫抑制干预解决。总体而言,10.2%的患者出现了≥3级的治疗相关不良事件,2.7%因治疗相关不良事件终止治疗。
结论
Durvalumab具有临床活性,可以为经历多种治疗的转移性NSCLC患者带来持续缓解。对于PD-L1表达≥25%的肿瘤,Durvalumab的活性更强。
Durvalumab耐受性可管理,该研究结果与其他抗PD-1/PD-L1抗体在转移复发性NSCLC中的表现相一致,未来可以进一步开展相关临床研究。
摘要原文
Results from the Phase 2 ATLANTIC Study:Durvalumab in ≥3rd–Line Locally Advanced or Metastatic, EGFR/ALK Wild–Type NSCLC
Background: Treatment with anti-PD-1/PD-L1 antibodies has demonstrated meaningful clinical benefit in patients with advanced NSCLC. Patients that progress after 2 lines of chemotherapy have few treatment options and poor outcomes. Durvalumab is an engineered human IgG1 mAb targeting programmed cell death ligand-1 (PD-L1).
Methods: ATLANTIC (NCT02087423) is a Phase 2, open-label, single-arm trial in patients with locally advanced or metastatic Stage IIIB–IV NSCLC (WHO PS 0 or 1; ≥2 prior systemic treatment regimens, including one platinum-based). There was no maximum number of prior treatments. The study initially enrolled all-comers and then was restricted to patients with PD-L1 high tumours (≥25% of tumour cells with membrane staining). The study includes three cohorts; here we report final results in Cohorts 2 and 3 that had EGFR/ALK wild-type or unknown status. Patients enrolled in Cohort 3 had ≥90% of tumour cells with PD-L1 staining. The primary endpoint is ORR (RECIST v1.1), based on independent central review. Secondary endpoints include DCR, DoR, PFS, OS, and safety (CTCAE v4.03).
Results: As of 3 June 2016, in Cohorts 2/3, 265/68 patients (median age 62/61 years, 67/72% PS 1, 21/29% squamous histology; mean of 3.2/2.6 prior therapies) had received durvalumab (10 mg/kg i.v. q2w). Responses were durable; in Cohort 2, patients with PD-L1 ≥25%, the ORR was similar in patients with squamous and non-squamous histology.
Most AEs were low grade and resolved with treatment delay and/or immunosuppressive interventions. Overall, 10.2% of patients had Grade ≥3 treatment-related AEs and 2.7% had treatment-related AEs leading to discontinuation.
Conclusion: Durvalumab was active and led to durable responses in a heavily pretreated metastatic NSCLC population; activity was numerically greater for patients whose tumours exceeded the 25% PD-L1 cutoff. The tolerability profile was manageable. Results are consistent with other anti-PD-1/PD-L1 therapies in metastatic, relapsed NSCLC and support further development of durvalumab.
