2016年12月4日-7日,第17届世界肺癌大会(WCLC)在奥地利维也纳盛大召开。会议进行到第4天(当地时间的7日上午),公布了4项重要免疫治疗研究成果。其中一项为美国Wayne州立大学Karmanos癌症研究所的Shirish Gadgeel教授的OAK研究,此次报告与ESMO上公布的结果相比,更新了研究的亚组分析数据。
研究背景
Atezolizumab是一种PD-L1抑制剂,阻断PD-L1对其受体PD-1和B7.1的结合。PD-L1抑制剂(注意不是PD-1)是从配体的角度,阻断负向调控信号,使T细胞恢复活性,从而增强抗肿瘤免疫应答。
Atezolizumab治疗经治的NSCLC的Ⅲ期研究OAK主要分析显示,在ITT人群中,Atezolizumab与多西他赛相比中位总生存(OS)分别为13.8 vs 9.6个月(HR=0.73);在PD-L1表达≥1%的患者(肿瘤细胞TC或肿瘤浸润免疫细胞IC)中,中位OS分别为15.7 vs 10.3个月(HR=0.74)。
在2016WCLC中,研究者报告了OAK研究的亚组分析。
研究内容
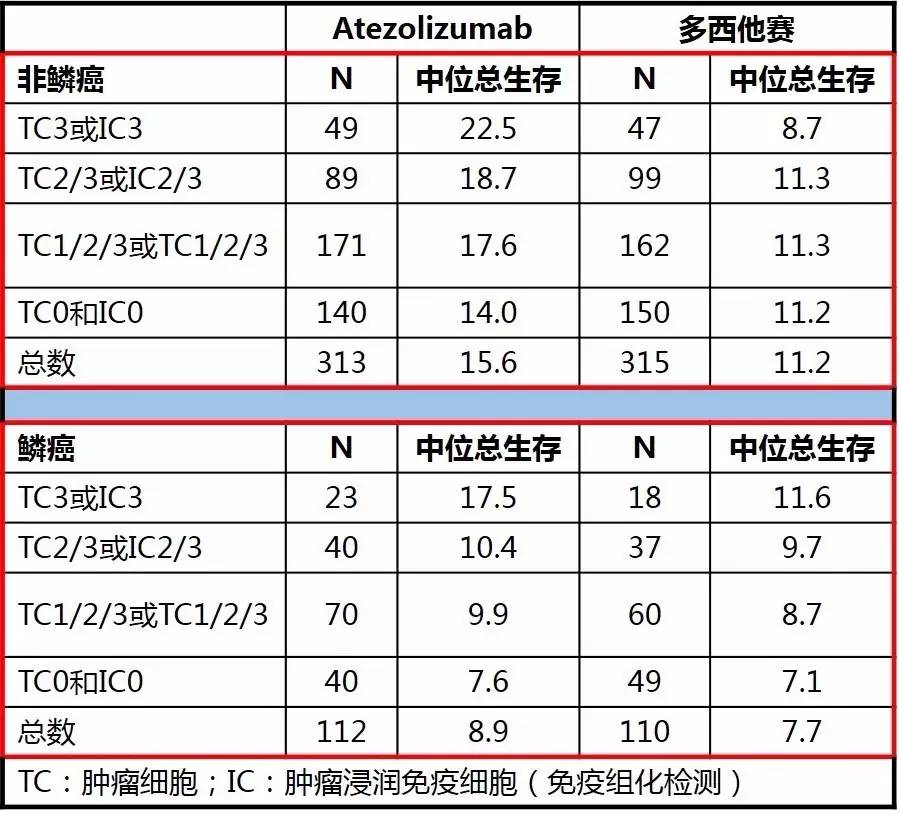
OAK研究在先前含铂化疗失败后的非选择NSCLC患者中,对比二线Atezolizumab和多西他赛的疗效和安全性。根据患者PD-L1的表达情况,先前的化疗方案和组织学进行分层,1:1随机将患者分配进Atezolizumab治疗(1200mg)或多西他赛(75mg/m2)治疗。
使用免疫组化和mRNA检测PD-L1表达情况,数据截至2016年7月7日。
研究结果
研究共纳入1225例NSCLC,对首批850例患者(主要研究人群)进行分析。相较于多西他赛而言,不论组织类型如何(鳞癌或非鳞癌)Atezolizumab都能够提供更多的生存改善;在不同的PD-L1表达亚组(TC或IC)中,Atezolizumab治疗的生存获益依然存在。
对于不同PD-L1检测手段,蛋白表达(IHC)和基因表达的相关患者总生存相似。
在非鳞癌患者中,Atezolizumab和多西他赛的客观缓解率(ORR)分别为14.4%vs 15.2%;在鳞癌患者中,ORR分别为11.6%vs 8.2%。
在基线存在脑转移的患者(n=85)中,Atezolizumab对比多西他赛的OS获益依然存在,分别为20.1 vs 11.9个月(HR=0.54);非吸烟患者中两治疗组的中位OS分别为16.3 vs 12.6个月(HR=0.71)。
结论
OAK研究显示,Atezolizumab在ITT人群中,包括所有组织亚型(不论PD-L1的表达情况)及其他包括不吸烟患者和具有基线脑转移患者的亚组,都具有临床获益。
摘要原文
OAK, a Randomized Ph III Study of Atezolizumab vs Docetaxel in Patients with Advanced NSCLC: Results from Subgroup Analyses
Background: Atezolizumab inhibits PD-L1 binding to its receptors PD-1 and B7.1, thereby restoring tumor-specific T-cell immunity. Primary analysis of the Phase III OAK study in previously-treated NSCLC revealed superior survival for atezolizumab vs docetaxel in the ITT population (mOS, 13.8 vs 9.6 months; HR, 0.73) and in patients expressing ≥1% PD-L1 on TC or IC (TC1/2/3 or IC1/2/3; mOS, 15.7 vs 10.3; HR, 0.74). Here we present further subgroup analyses.
Methods: OAK evaluated atezolizumab vs docetaxel in an unselected NSCLC population who had failed prior platinum-containing chemotherapy. Patients were stratified by PD-L1 expression, prior chemotherapy regimens and histology, and randomized 1:1 to atezolizumab (1200 mg) or docetaxel (75 mg/m[2]) IV q3w. PD-L1 expression by IHC and mRNA was centrally evaluated by VENTANA SP142 IHC assay and Fluidigm, respectively. Data cutoff, July 7, 2016.
Results: For the first 850 of 1225 randomized patients (primary study population), OS was improved with atezolizumab vs docetaxel regardless of histology and this benefit was observed across PD-L1 subgroups within each histology (Table). PD-L1 gene expression showed a similar association with OS as PD-L1 IHC. In nonsquamous patients ORR was 14.4% vs 15.2%; in squamous patients ORR was 11.6% vs 8.2% (atezolizumab vs docetaxel). OS benefit vs docetaxel was seen across subgroups including patients with treated baseline brain metastases (n=85; mOS 20.1 vs 11.9 mo; HR 0.54, 95% CI 0.63-0.89) and never smokers (n=156; mOS 16.3 vs 12.6 mo, HR 0.71, 95% CI 0.47-1.08). Further secondary endpoints and exploratory biomarker analyses for these subgroups and by age and EGFR/KRAS status will be presented.
Conclusion: OAK demonstrated clinically relevant improvements with atezolizumab in the ITT population, including in both histology subgroups regardless of PD-L1 expression (measured by IHC or tumor gene expression), and among other subgroups including never smokers and in patients with baseline brain metastases.
