医脉通编译整理,转载请务必注明出处。
2016年美国临床肿瘤学会(ASCO)年会将于6月3日至7日在美国芝加哥举行。一项关于PD-1抑制剂pembrolizumab为晚期黑色素瘤患者带来长期生存获益的报告将在会上公布(摘要号9503)。医脉通带您先睹为快。
ASCO观点
ASCO发言人Don S. Dizon评论表示:“阻断PD-1的新疗法正在延长许多患者的生存,可能会给一些晚期黑色素瘤患者提供比以往更长的生存。在几年时间内,这些治疗将会真正改变黑色素瘤及其许多其它难以治疗的癌症的局面。”
研究详情
据一项1b期研究KEYNOTE-001的长期随访结果,在新诊断和经治的晚期黑色素瘤患者中,40%的患者在开始pembrolizumab治疗后的第三年仍然存活,两组患者的总生存率相似,均为36个月。
基于KEYNOTE-001的数据,Pembrolizumab于2014年9月获批用于晚期黑色素瘤的治疗。其它的临床研究(KEYNOTE-002和KEYNOTE-006)也显示与化疗或伊匹单抗相比,pembrolizumab对晚期黑色素瘤患者具有生存获益。
值得注意的是,根据免疫相关缓解标准,该研究15%的患者经历了完全缓解。在这些患者中,89%的患者仍在缓解中。在2011年之前,当伊匹单抗被批准作为首个用于延长黑色素瘤患者生存的药物时,晚期黑色素瘤患者的中位总生存不足1年。
研究的第一作者Caroline Robert、巴黎Gustave-Roussy研究所血液肿瘤学主任Caroline Robert表示:“晚期黑色素瘤仍然是非常具有挑战性的癌症,这就是获得长期生存获益的这项大型研究如此了不起的原因。这项研究的结果进一步显示了pembrolizumab取得长期获益的潜力。“
重要发现
这项I期研究纳入了655例确诊为晚期黑色素瘤的患者。75%的患者之前接受过其它治疗,包括伊匹单抗。患者每3周接受2或10 mg/kg的pembrolizumab,或每2周10 mg/kg。在试验期间,每三周2 mg/kg的剂量被认定为最佳剂量。患者接受治疗直至疾病进展,出现不可耐受毒性或研究人员做出决定。
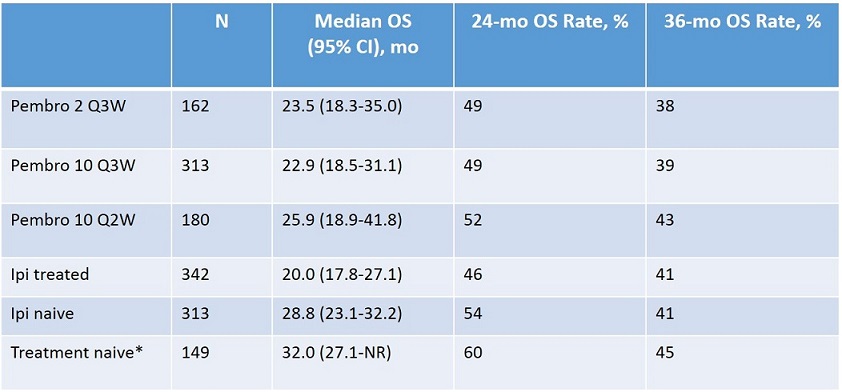
接受pembrolizumab治疗的患者的3年总生存率为40%,中位总生存为24.4个月。
基于之前的黑色素瘤治疗,患者的生存率稍有不同。未经治疗的患者的生存率更高一些,为45%。经伊匹单抗治疗和未经该药治疗的患者之间的3年生存率相似(为41%)。Pembrolizumab治疗的平均治疗时间为11.3个月。在达到了完全缓解后,总共有61位(9%)患者停止pembrolizumab治疗,97%的患者在分析时仍处在缓解中。对于停止pembrolizumab治疗后仍保持缓解的患者,停药后的中位缓解时间为10个月或仍未达到。据研究人员表示,尽管基于这项单臂早期研究很难得出确定性结论,但是这些鼓舞人心的数据显示,无论患者之前是否接受过治疗,都可以从pembrolizumab中获益。
总之,pembrolizumab耐受良好,安全性和耐受性与其它大型临床试验中观察到的一致。与治疗相关的最常见的不良事件为疲劳(40%),皮肤发痒(28%),和皮疹(23%),仅有8%的患者由于治疗相关副反应而停药。
摘要原文
Abstract 9503: 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001.
Background: The anti–PD-1 antibody pembro (pembro; MK-3475) prevents PD-1 from binding to its ligands, PD-L1 and PD-L2, and is approved for treating advanced melanoma at a dose of 2 mg/kg every 3 wk (Q3W). Pembro demonstrated superior PFS over chemotherapy for ipilimumab (ipi)-refractory melanoma (KEYNOTE-002) and superior OS and PFS over ipi for advanced melanoma (KEYNOTE-006). We present 3-year OS data for all patients (pts) with melanoma enrolled in the phase 1b KEYNOTE-001 study (NCT01295827).
Methods: Pts were enrolled in ipi-naive and ipi-treated cohorts and received pembro 2 or 10 mg/kg Q3W or 10 mg/kg Q2W until intolerable toxicity, progression, or investigator decision. Clinically stable pts with radiographic progression could remain on pembro until progression was confirmed. Response was assessed by RECIST v1.1 every 12 wk. After pembro discontinuation, pts were contacted to assess survival every 3 mo. OS was estimated using the Kaplan-Meier method.
Results: Of the 655 pts enrolled, 24% had BRAFV600mutation, 78% had stage M1c disease, 38% had elevated LDH, 75% had ≥1 prior therapy, and 52% had prior ipi. As of the Sep 18, 2015, data cutoff date, median follow-up duration was 32 mo (range 24-46) and 358 (55%) pts had died. The 36-mo OS rate was 40% and median OS was 23.8 mo (95% CI, 20.2-29.0), with similar results for each dose (Table). 36-mo OS rates were 41% in both ipi-treated and ipi-naive pts and 45% in treatment-naive pts (Table). Examination of the OS curve suggests a long-term OS benefit for a fraction of pts treated with pembro. Additional data, including PFS, ORR, duration of response, and safety, will be available for presentation.
Conclusions: Pembro provides long-term survival benefit in pts with ipi-naive and ipi-treated advanced melanoma, with 40% of pts alive at 3 years. These data support the use of pembrolizumab in pts with advanced melanoma regardless of prior treatment.
