医脉通编译,转载请务必注明出处
2016年6月3-7日,一年一度的美国临床肿瘤学会(American Society of Clinical Oncology,ASCO)年会将在芝加哥举办。6月5日上午的消化系统(结直肠)肿瘤口头报告专场上,一项摘要号为3501的CheckMate-142中期试验,将Nivolumab±ipilimumab治疗高度微卫星不稳定(MSI-H)的转移性结直肠癌(mCRC)患者和非-MSI-H表型的mCRC患者的数据进行讨论,医脉通整理如下:
目前证据支持nivolumab(N)用于MSI-H表型mCRC的治疗。Nivolumab,一种全人源化抗-PD-1单克隆抗体和ipilimumab(I),一种人源化抗-CTLA-4单克隆抗体,在其他肿瘤中有良好的安全性和有效性。CheckMate-142,一项2期研究,在MSI-H表型和非-MSI-H表型的mCRC患者中对N±I疗效进行评估。
患者ECOG PS评分为0-1,同时对至少1次以上治疗不耐受/进展。MSI-H表型mCRC患者接受N 3mg/kg q2 wk(N3)或者N 3mg/kg+I 1mg/kg q3 wk(N3+I1)×4次剂量序贯N3直到疾病进展(PD)或者其他原因停药。N+I3次剂量的初步评估在非-MSI-H表型mCRC患者中完成。主要终点是研究人员根据RECIST 1.1报道的ORR;其他终点是安全性,OS,和PFS。
一共有33例(N3)和26例(N3+I1)MSI-H表型患者,和3例(N1+I1),10例(N1+I3),和10例(N3+I1)的非-MSI-H表型患者入组。82%(N3)和92%(N3+I1)的MSI-H表型以及100%的非-MSI-H表型患者接受过≥2次方案。15%(N3)和25%(N3+I1)的MSI-H表型患者是已知的BRAF V600E。17例(52%;N3)和19例(73%;N3+I1)MSI-H表型患者仍在治疗中。有效性结果如表所示。
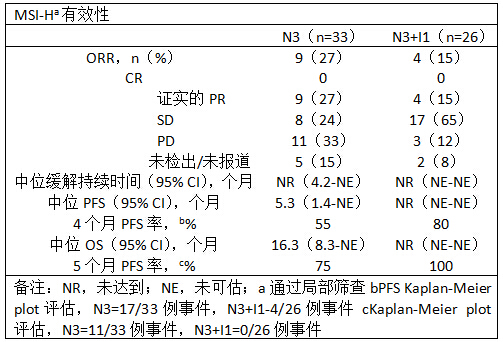
在MSI-H表型患者中,治疗相关的不良事件(TRAEs)发生在26例(79%;N3)和22例(85%;N3+I1)患者中;最常见的是腹泻和疲劳(每种27%;N3)以及腹泻(46%;N3+I1)。3-4级TRAEs发生在7例(N3)和8例(N3+I1)患者中。1例接受N3的患者出现5级TRAE(猝死)。在非-MSI-H表型患者中中位PFS(95% CI)是1.4个月(1.2-1.9;汇总N+I)。
综上所述,在大多数患者中,Nivolumab和Nivolumab+ipilimumab方案耐受性良好,并在MSI-H表型mCRC患者中表现出可喜的临床活性和生存期。这项研究正在进行中。临床试验信息:NCT02060188。
会议专题》》》2016年ASCO年会专题报道
原文摘要:
Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results(Abstract3501)
Authors:Michael J.Overman, Scott Kopetz,et al.
Session Type:Oral Abstract Session
Background: Evidence supports use of nivolumab (N) in MSI-H mCRC. N, a fully human anti-PD-1 mAb and ipilimumab (I), a humanized anti-CTLA-4 mAb, have favorable safety & efficacy in other tumors. CheckMate-142, a phase 2 study, evaluates N ± I in pts with mCRC, MSI-H and non-MSI-H.
Methods: Pts had ECOG PS 0–1, and intolerance/progression on ≥ 1 tx. MSI-H pts received N 3 mg/kg q2 wk (N3) or N 3 mg/kg + I 1 mg/kg q3 wk (N3+I1) x 4 doses followed by N3 until disease progression (PD) or other discontinuation. Initial evaluation of N+I at 3 doses was completed in non-MSI-H pts. Primary endpoint was investigator-reported ORR by RECIST 1.1; other endpoints were safety, OS, and PFS.
Results: 33 (N3) and 26 (N3+I1) MSI-H pts, and 3 (N1+I1), 10 (N1+I3), and 10 (N3+I1) non-MSI-H pts were enrolled. 82% (N3) and 92% (N3+I1) of MSI-H and 100% of non-MSI-H pts had ≥ 2 prior regimens. 15% (N3) and 25% (N3+I1) of MSI-H pts had known BRAF V600E. 17 (52%; N3) and 19 (73%; N3+I1) MSI-H pts remain on tx. Efficacy results are shown in the Table. In MSI-H pts, tx-related adverse events (TRAEs) occurred in 26 (79%; N3) and 22 pts (85%; N3+I1); most common were diarrhea and fatigue (27% each; N3) and diarrhea (46%; N3+I1). Grade 3–4 TRAEs occurred in 7 (N3) and 8 pts (N3+I1). One pt on N3 had a Grade 5 TRAE (sudden death). In non-MSI-H pts median (95% CI) PFS was 1.4 mo (1.2–1.9; pooled N+I).
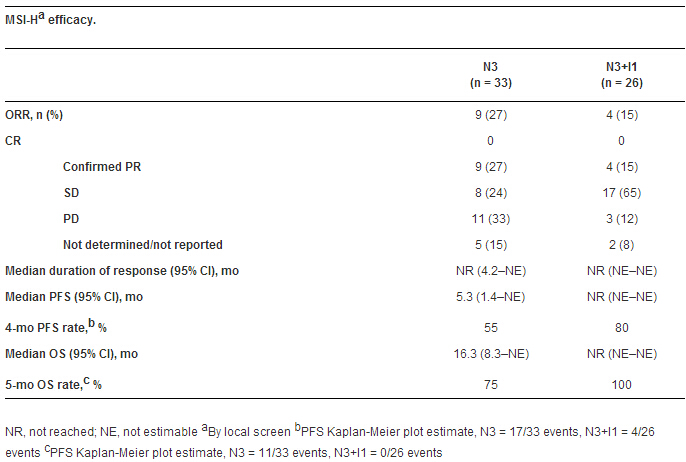
Conclusions: N and N+I were well tolerated in most pts and demonstrated encouraging clinical activity and survival in MSI-H mCRC. This study is ongoing. Clinical trial information: NCT02060188
打开微信 →→ 添加“医脉通肿瘤科”公众号,或扫描电脑屏幕右上方二维码 →→ 关注医脉通肿瘤科。随时随地获取肿瘤前沿资讯,一次打包最实用的肿瘤治疗知识。做科研达人、临床高手,尽在医脉通肿瘤频道。
