索拉非尼是一种多激酶抑制剂,已证明对不能手术切除的肝细胞癌有效。研究人员对肝细胞癌(HCC)患者给予辅助索拉非尼的疗效和安全性进行评估。该项STORM试验,是用来评估使用辅助索拉非尼预防肝细胞癌复发的一项III期随机,双盲,安慰剂对照试验,下面和大家提前分享这项研究。
研究方法:
以根治性为目的接受手术切除或者局部消融,以及有中级或者高级复发风险的患者均符合该试验要求。主要入选标准包括Child-Pugh评分5~7,ECOG PS 0,经CT或者MRI确认没有残留病灶。排除标准为复发性HCC,腹水,肝外转移,大血管浸润,和先前接受HCC系统治疗。按照根治性治疗方式,地理区域,复发风险,和Child-Pugh状态将患者进行分层,以1:1比例将患者分为接受索拉非尼400mg 口服 每日两次或者安慰剂,最长维持时间4年。根据独立审查主要终点是无复发生存期(RFS)。次要终点包括复发时间(TTR)和总生存期(OS)。
研究结果:
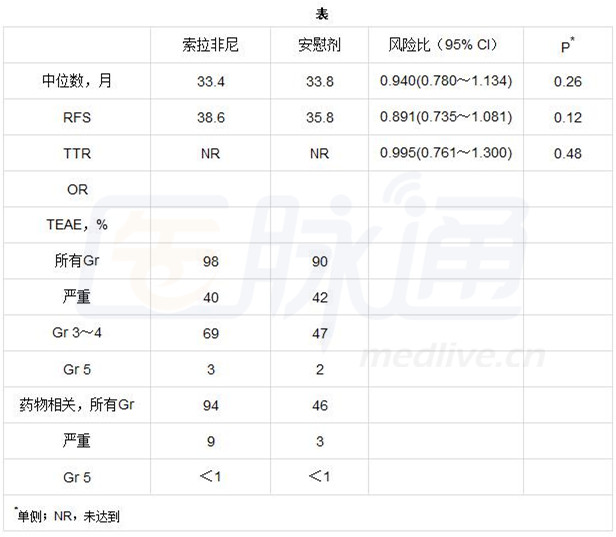
共有1114例患者被随机分配到索拉非尼组(n=556)和安慰剂组(n=558)。两组之间的基线特征被平衡。中位年龄是59岁,62%的亚洲人,81%接受手术切除术,97%为Child-Pugh A,并且有46%存在高复发风险。基于464例RFS事件进行分析。RFS,TTR和OS观察到没有明显差异(表)。索拉非尼组有一个较短的中位治疗维持时间(12.5个月 vs 22.2个月),以及较低的中位日常剂量(578mg vs 778mg)。由于治疗中出现的不良反应事件(TEAE)索拉非尼组的停药率较高(24% vs 7%),同意撤出试验(17% vs 6%)。最常见的3~4级(Gr)TEAEs发生在索拉非尼组更为频繁,与安慰剂组比较,主要是手足皮肤反应(28%),高血压(7%),和腹泻(6%)。
研究结论:
该试验的主要终点未能满足。不良反应与已知的索拉非尼安全特性相一致,没有新的安全性结果被观察到。
医脉通整理报道,转载请注明出处。
会议专题》》》2014年ASCO年会专题报道
阅读原文摘要
STORM: A phase III randomized,double-blind,placebo-controlled trial of adjuvant sorafenib after resection or ablation to prevent recurrence of hepatocellular carcinoma (HCC).(Abstracts4006^)
Authors: Jordi Bruix, Tadatoshi Takayama, Vincenzo Mazzaferro, et al.
Session Type: General Poster Session
Background: Sorafenib is a multikinase inhibitor with proven efficacy in unresectable HCC. We evaluated the efficacy and safety of adjuvant sorafenib for HCC.
Methods: Patients who had undergone surgical resection or local ablation with curative intent and had an intermediate or high recurrence risk were eligible. Main inclusion criteria were Child-Pugh score 5–7, ECOG PS 0, and no residual disease confirmed by CT or MRI. Exclusion criteria included recurrent HCC, ascites, extrahepatic spread, macrovascular invasion, and prior systemic therapy for HCC. Patients were stratified by curative treatment, geographic region, recurrence risk, and Child-Pugh status and randomized 1:1 to treatment with sorafenib 400 mg orally twice a day or placebo for a maximum of 4 yrs. The primary endpoint was recurrence-free survival (RFS) by independent review. Secondary endpoints included time to recurrence (TTR) and overall survival (OS).
Results: A total of 1114 patients were randomized (n=556 sorafenib; n=558 placebo). Baseline characteristics were balanced between groups. Median age was 59 yrs, 62% were Asian, 81% had resection, 97% were Child-Pugh A, and 46% had high recurrence risk. The analysis was based on a total of 464 RFS events. No differences in RFS, TTR, and OS were observed (Table). The sorafenib group had a shorter median treatment duration (12.5 vs 22.2 mos) and lower mean daily dose (578 vs 778 mg). Discontinuation rates with sorafenib were higher due to treatment-emergent adverse events (TEAE) (24% vs 7%) and consent withdrawal (17% vs 6%). Most common grade (Gr) 3–4 sorafenib TEAEs occurring more frequently vs placebo were hand-foot skin reaction (28%), hypertension (7%), and diarrhea (6%).
Conclusions:The primary endpoint of the trial was not met. Adverse events are consistent with the known sorafenib safety profile and no new safety findings were observed. Clinical trial information: NCT00692770.
