尽管局部晚期胃癌辅助化疗取得了进展,但是其预后仍然较差。研究人员开展了一项胃癌辅助多中心小组试验(SAMIT)以评估序贯治疗(紫杉醇序贯替加氟+尿嘧啶[UFT]或者紫杉醇序贯S-1)对比单药治疗(UFT或者S-1)的优越性,同时也进行了UFT对比S-1的非劣效性。该项研究于6月19日在线发表于《Lancet Oncology》上。
研究人员在日本的230家医院开展了一项2×2析因设计随机III期试验。他们招募了20~80年龄范围内的T4a或者T4b胃癌患者,这些患者已经接受D2清扫术,ECOG评分0~1。患者根据肿瘤大小,淋巴结转移,和研究位置随机分配到四个治疗组中。患者只接受UFT(267mg/m2每天),或S-1(80mg/m2每天),维持14天,有一个7天的歇药周期,或者间歇性每周紫杉醇(80mg/m2)三个周期序贯UFT或者S-1。在单药治疗组中治疗持续48周,序贯治疗组为49周。主要终点是无疾病生存期,采用意向治疗进行评估。我们评估UFT是否不劣于S-1,有一个1.33的非劣效性界限。该项试验在UMIN临床试验登记注册,编号C000000082。
在该项试验中,研究者将2004年8月3日到2009年9月29日之间的1495例患者进行随机分配。只接受UFT治疗的患者374例,只进行S-1治疗的患者374例,374例患者接受紫杉醇序贯UFT,373名患者接受紫杉醇序贯S-1。在初步分析中纳入1433例患者经过至少3年的随访期(每组分别为:359,364,355,355)。完成协议治疗的患者分别为:UFT组215名(60%),S-1组224名(62%),紫杉醇序贯UFT组242名(68%),紫杉醇序贯S-1组250名(70%)。
单药治疗组的3年无疾病存活率是54.0%(95% CI 50.2~57.6),序贯治疗组为57.2%(53.4~60.8;风险比[HR]0.92,95% CI 0.80~1.07,P=0.273)。UFT组3年无疾病存活率是53%(95% CI 49.2~59.6),S-1组是58.2%(54.4~61.8;HR 0.81,95% CI 0.70~0.93,P=0.0048;P非劣效性=0.151)。
最常见3~4级血液学不良反应是中性粒细胞减少(详情见表),最常见的3~4级非血液学不良反应为食欲不振。
表
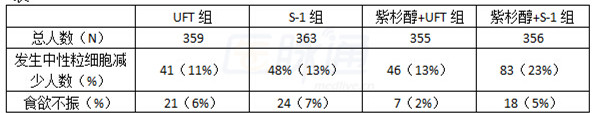
通过该项试验,研究人员认为序贯治疗不能提高无疾病生存期,UFT并不非劣效于S-1(即S-1优于UFT),因此S-1单药治疗仍然作为晚期胃癌的标准治疗方案。
医脉通编译自:Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial,Lancet Oncology,19 June 2014
摘要原文:
Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial
Background
The prognosis for locally advanced gastric cancer is poor despite advances in adjuvant chemotherapy. We did the Stomach cancer Adjuvant Multi-Institutional group Trial (SAMIT) to assess the superiority of sequential treatment (paclitaxel then tegafur and uracil [UFT] or paclitaxel then S-1) compared with monotherapy (UFT or S-1) and also the non-inferiority of UFT compared with S-1.
Methods
We did this randomised phase 3 trial with a two-by-two factorial design at 230 hospitals in Japan. We enrolled patients aged 20-80 years with T4a or T4b gastric cancer, who had had D2 dissection and a ECOG performance score of 0-1. Patients were randomly assigned to one of four treatment groups with minimisation for tumour size, lymph node metastasis, and study site. Patients received UFT only (267 mg/m2 per day), S-1 only (80 mg/m2 per day) for 14 days, with a 7-day rest period or three courses of intermittent weekly paclitaxel (80 mg/m2) followed by either UFT, or S-1. Treatment lasted 48 weeks in monotherapy groups and 49 weeks in the sequential treatment groups. The primary endpoint was disease-free survival assessed by intention to treat. We assessed whether UFT was non-inferior to S-1 with a non-inferiority margin of 1.33. This trial was registered at UMIN Clinical Trials Registry, number C000000082.
Findings
We randomly assigned 1495 patients between Aug 3,2004,and Sept 29,2009.374 patients were assigned to receive UFT alone, 374 to receive S-1 alone, 374 to received paclitaxel then UFT, and 373 to receive paclitaxel then S-1. We included 1433 patients in the primary analysis after at least 3 years of follow-up (359, 364, 355, and 355 in each group respectively). Protocol treatment was completed by 215 (60%) patients in the UFT group, 224 (62%) in the S-1 group, 242 (68%) in the paclitaxel then UFT group, and 250 (70%) in the paclitaxel then S-1 group. 3-year disease-free survival for monotherapy was 54.0% (95% CI 50.2—57.6) and that of sequential treatment was 57.2% (53.4—60.8; hazard ratio [HR] 0.92, 95% CI 0.80—1.07, p=0.273). 3-year disease-free survival for the UFT group was 53.0% (95% CI 49.2—56.6) and that of the S-1 group was 58.2% (54.4—61.8; HR 0.81, 95% CI 0.70—0.93, p=0.0048; pnon-inferiority=0.151). The most common grade 3—4 haematological adverse event was neutropenia (41 [11%] of 359 patients in the UFT group, 48 [13%] of 363 in the S-1 group, 46 [13%] of 355 in the paclitaxel then UFT group, and 83 [23%] of 356 in the paclitaxel then S-1 group). The most common grade 3—4 non-haematological adverse event was anorexia (21 [6%], 24 [7%], seven [2%], and 18 [5%], respectively).
Interpretation
Sequential treatment did not improve disease-free survival, and UFT was not non-inferior to S-1 (and S-1 was superior to UFT), therefore S-1 monotherapy should remain the standard treatment for locally advanced gastric cancer in Japan.
