医脉通编译整理,转载请务必注明出处。
2015年ASCO会议的脚步越来越近了。计划于6月1日上午进行的乳腺癌口头报告专场上,米兰San Raffaele IRCCS医院的Luca Gianni等将带来对2期研究NeoSphere的5年分析结果。该研究评估了4个周期的多西他赛(D)和/或曲妥株单抗(T)和/或帕妥珠单抗(P)新辅助治疗的疗效(摘要号505)。医脉通带您提前了解下这项研究。
在NeoSphere研究中,评估了4个周期的曲妥株单抗(T)+多西他赛(D), 帕妥珠单抗(P)+T+D,P+T, 或P+D治疗,随后进行手术和辅助化疗加传统曲妥株单抗治疗对417位局部晚期的、炎性或早期HER2阳性乳腺癌患者的疗效。将帕妥珠单抗加入到曲妥珠单抗联合多西他赛的治疗中,使乳房的病理完全缓解率(pCR)提高了16.8%(bpCR, ypT0/; 首要终点),并使乳房和腋窝的总pCR增加了17.8% (tpCR, ypT0/, ypN0),这一数字不但有统计学显著差异,而且还具有临床意义。
对最后一位患者进行随机分配后,进行了计划前治疗意向分析,以评估无病生存(DFS;从手术至进展或死亡的时间),无进展生存(PFS;从随机分配到进展或死亡的时间,等同于通常无事件生存的定义)。
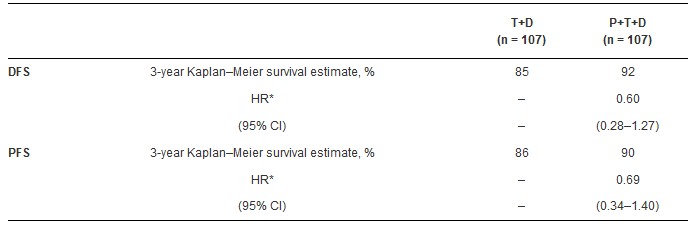
下表显示了P+T+D方案与T+D相比,在三年的生存率,风险比,及95% CI方面的差异。在P+T和P+D组中,3年的DFS分别为88%和84%,PFS分别为81%和82%。在对四组的合并分析中,所有获得tpCR的患者与所有未获得tpCR的患者相比,DFS的HR为0.68 (95% CI, 0.36–1.26),PFS的HR为0.54 (95% CI, 0.29–1.00)。
Compared with T+D
长期结果即3年生存率,与首要终点(bpCR)的结果相一致。结果表明尽管P+T+D及T+D 组中的辅助治疗方案相似,但是将帕妥珠单抗加入到曲妥株单抗联合多西他赛的方案后,所获得的收益可以持续。这些结果也支持了pCR与长期结果改善之间的联系。
摘要原文(摘要号505)
Background: InNeoSphere, four cycles of T+D, P+T+D, P+T, or P+D, followed by surgery and adjuvant chemotherapy plus conventional T, were evaluated in 417 women with locally advanced, inflammatory, or early HER2-positive breast cancer. The addition of P to T+D led to a statistically significant and clinically meaningful 16.8% increase (95% confidence interval [CI], 3.5–30.1; P = .0141) in pathologic complete response rate (pCR) in the breast (bpCR, ypT0/is; primary endpoint), and a 17.8% increase in total pCR in the breast and axilla (tpCR, ypT0/is, ypN0).
Methods: A pre-planned descriptive intent-to-treat analysis was conducted 5 years after randomization of the last patient, to evaluate disease-free survival (DFS; time from surgery until progression or death) and progression-free survival (PFS; time from randomization until progression or death, equivalent to the commonly used definition of event-free survival).
Results: Three-year survival rates, hazard ratios (HRs), and 95% CIs for the main analysis of P+T+D compared with T+D are summarized in the table. In the P+T and P+D arms, respectively, 3-year survival rates were 88% and 84% for DFS, and 81% and 82% for PFS. Across all four treatment arms pooled, for all patients who achieved tpCR versus all patients who did not achieve tpCR, the HR for DFS was 0.68 (95% CI, 0.36–1.26) and the HR for PFS was 0.54 (95% CI, 0.29–1.00).
Conclusions: Longer-term outcomes as defined by three-year survival rates, are in line with the results of the primary endpoint (bpCR), suggesting a benefit of P added to T+D that persists over time despite use of identical adjuvant therapy in the P+T+D and T+D arms. These results also support the association between pCR and improvements in long-term outcomes. Clinical trial information: NCT00545688
更多ASCO精彩内容》》》2015年ASCO年会专题报道
