医脉通整理,转载请务必注明出处。
2016年6月3日-7日,美国临床肿瘤学会(ASCO)2016年会在美国芝加哥盛大召开。当地时间5号下午1点,本次年会最令人期待的全体大会开幕,会上来自麻省总医院癌症中心的Paul E. Goss教授公布了乳腺癌芳香化酶抑制剂(Aromatase Inhibitor, AI)治疗的MA.17R研究结果。MA.17R研究从5000多项入选摘要中脱颖而出,得到全体大会汇报机会,足见其重要性。

全体大会现场
曾经轰动一时MA.17研究入组了5000多名绝经后早期乳腺癌患者,通过来曲唑和安慰剂对比证实AI带来的临床获益。MA.17研究结论说明来曲唑是自TAM被证明能够改善总生存率后,20年来又一个被证明对总生存率有积极影响的内分泌药物。
对于绝经后、雌激素阳性的早期乳腺癌患者,辅助AI直接治疗5年,或跟随他莫昔芬(TAM)治疗后AI再用5年都是标准治疗。如果在此基础上AI继续用5年能够降低复发风险吗?(LBA1),长期加用来曲唑是否会对患者的生活质量产生负面影响?(LBA506)。MA.17R研究承前启后,在较大的样本上求证了这两个问题。
主要研究内容
MA.17R是由加拿大癌症试验工作组领导的一项随机双盲,安慰剂对照的Ⅲ期临床试验。
研究方案设计:
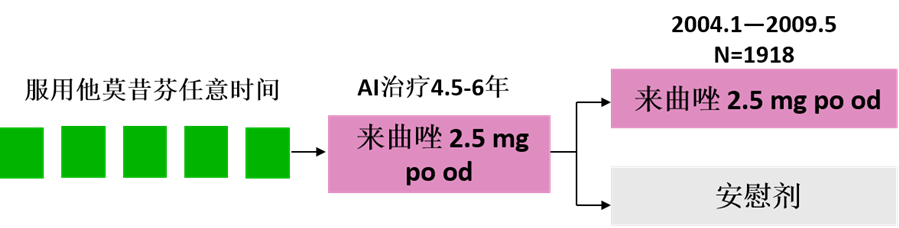
患者入组主要标准:
◆ER+/PR+乳腺癌
◆绝经后,无疾病存在
◆完成4.5-6年辅助AI治疗
◆曾接受任意时间的TAM治疗
◆预期寿命至少≥5年
主要终点是无病生存期(DFS),次要终点是总体生存(OS)、安全性、对侧乳腺癌(CBC)和生存质量(QOL)。
使用标准化SF-36健康调查量表和绝经期生存质量量表(MENQOL)评估入组乳腺癌患者的QOL。
主要研究结果
研究纳入1918名乳腺癌患者,中位随访时间75个月(6.3年)。
1. LBA1:
DFS事件在AI治疗组和安慰剂组分别出现67vs 98,其中癌症复发次数在两组中分别为42 vs 53。
◆在随访时间内,AI组和安慰剂组均死亡100例。
◆5年DFS率在AI组和安慰剂组分别为95% vs 91%,AI组乳腺癌复发风险降低34%。
◆5年OS率在AI组和安慰剂组分别为93% vs 94%,无明显差异。
◆CBC年发生率在AI组和安慰剂组分别为0.21% vs 0.49%。
用药副作用方面,骨折出现在AI组和安慰剂组分别为133例 vs 88例;新发骨质疏松出现在两组分别为109例 vs 54例。
2. LBA506:
共1428名乳腺癌患者完成基线QOL评估,而QOL评价的依从性超过85%。
◆基线SF-36躯体评价(PCS)总分在AI组和安慰剂组分别为47.5 vs 47.9,SF-36精神评价(MCS)总分在两组分别为55.5 vs 54.8,均接近正常人群。
◆AI组和安慰剂组的SF-36(PCS和MCS)和MENQOL量表平均得分无明显差异。
◆AI组患者与安慰剂组相比,血管舒缩症状较重,性功能受到更多影响。
主要结论
雌激素阳性的早期乳腺癌患者面临着不确定的复发风险,MA.17R研究证实了延长AI治疗可以降低乳腺癌复发风险,更长的AI治疗对CBC可以起到实质性的预防作用。
本研究未发现长时间服用来曲唑会带来新的毒副作用,但是骨健康依然是平衡用药获益和风险的主要考量。
MA.17R研究中AI治疗组和安慰剂组患者总体生存无显著性差异,主要原因是雌激素受体阳性乳腺癌缓慢复发的自然特点。基于对无疾病生存期的改善,内分泌治疗在乳腺癌临床应用广泛。另一方面,早期乳腺癌的生存长,需要关注长期内分泌治疗患者的生活质量。本研究中AI组和安慰剂组总体上QOL并无明显的临床差异,说明延长来曲唑治疗并未对患者的生活产生严重的不良影响,但需要注意血管舒缩症状和性功能。
专家评论
MA.17R能告诉我们什么?
◆即使是在确诊15年后,还是有一小部分早期乳腺癌患者会复发
◆在他莫昔芬治疗之后,10年的来曲唑治疗能使约3.2%的患者取得DFS改善
◆在长达10年的治疗中,没有出现更多的药物副作用
但是,来曲唑治疗患者DFS改善的主要贡献来自于对侧乳腺癌发生率下降。而远处复发在AI组和安慰剂组中的差异只有1.1%,目前没有显著的生存获益。此外,研究数据也不支持新辅助治疗。
不同观点
◆结合临床和基因组学参数建立新的模型来预测乳腺癌远期复发风险,避免在不必要的人群中过度使用内分泌治疗
◆从其他研究中获取更多的数据来证实来曲唑的效用
◆5年的额外治疗增加了花费以及骨折、骨质疏松的风险,现在还不能轻易给予门诊患者长达10年之久来曲唑治疗
◆对于已摆脱服药早期副作用影响的高风险患者,应该告诉他们来曲唑的作用并帮助他们做出用药选择。
现阶段延长AI治疗研究
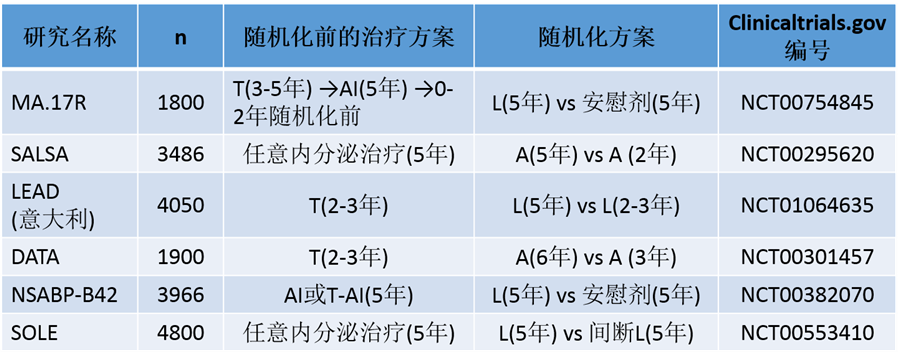
A: 阿那曲唑;AI:芳香化酶抑制剂;L:来曲唑;T:他莫昔芬
LEAD :Letrozole Adjuvant Therapy Duration trial
SALSA:Secondary Adjuvant Long-term Study with Arimidex trial
DATA:Different Durations of Anastrozole afterTamoxifen trial
SOLE:Study of Letrozole Extensiontrial
2016ASCO年会精彩不断,关注医脉通肿瘤科,后续会有更多国内肿瘤学界大咖的采访实录~~
会议专题》》》2016年ASCO年会专题报道
摘要阅读
AbstractNo:LBA1
A randomized trial (MA.17R) of extendingadjuvant letrozole for 5 years after completing an initial 5 years of aromataseinhibitor therapy alone or preceded by tamoxifen in postmenopausal women withearly-stage breast cancer
Session: Plenary Session Including the Science of Oncology Award and Lecture
Type: Plenary Session
Author(s): Paul E. Goss, James N. Ingle, Kathleen I. Pritchard, et al.
Background: Five years of aromatase inhibitor (AI) therapyeither as up-front treatment or after 2-5 years of tamoxifen has become thestandard of care for postmenopausal women with hormone receptor positive earlybreast cancer. Extending treatment with an AI to 10 years may further reducethe risk of breast cancer recurrence.
Methods: We conducted a double-blind, placebo-controlled trial (CanadianCancer Trials Group MA.17R) to test the efficacy of extending AI treatment foran additional five years using letrozole. The primary endpoint was disease-freesurvival.
Results: A total of 1,918 women with early stage breast cancer were enrolled(median follow-up 75 months, 6.3 years). A total of 165 disease-free survival(DFS) events (67 on letrozole and 98 on placebo) occurred, of which 42 versus53 were distant recurrences on letrozole and placebo, respectively. There were200 deaths (100 in each treatment group). The 5 year DFS was respectively 95%for patients receiving letrozole versus 91% for those on placebo (HR 0.66; P= 0.01)from a two-sided log-rank test stratified by nodal status, prior adjuvantchemotherapy, interval between last dose of AI therapy and randomization, andduration of prior tamoxifen at randomization. The 5 year overall survival wasrespectively 93% for subjects on letrozole and 94% on placebo with a HR of 0.97(P=0.83). The annual incidence rate of contralateral breast cancer was 0.21%for subjects on letrozole versus 0.49% on placebo (P=0.007).
Conclusions: Compared to 5 years of AI treatment as initial therapy or precededby 2-5 years of tamoxifen, extending AI treatment to 10 years significantlyimproves disease-free survival. Further analyses will provide a comprehensivepicture of toxicities and QOL.
AbstractNo:LBA506
Patient-reported outcomes from MA.17R: Arandomized trial of extending adjuvant letrozole for 5 years after completingan initial 5 years of aromatase inhibitor therapy alone or preceded bytamoxifen in postmenopausal women with early-stage breast cancer
Session: Breast Cancer—HER2/ER
Type:Oral Abstract Session
Author(s):Julie Lemieux, Paul E. Goss, Wendy R. Parulekar, etal.
Background: MA.17R is a Canadian Cancer Trials Group(CCTG) led phase III randomized controlled trial comparing letrozole to placeboafter 5 years of aromatase inhibitor (AI) as adjuvant therapy forhormone-receptor positive breast cancer. The primary endpoint was disease-freesurvival. Quality of life (QOL) was a secondary endpoint.
Methods: QOL was measured with the SF-36 (2 summaryscores and 8 domains) and menopause-specific QOL (MENQOL) (4 symptom domains)at baseline and every 12 months (mo) up to 60 mo. QOL assessment was mandatoryfor CCTG centres but optional to centres in other groups. Mean change scoresfrom baseline were calculated at 12 and 36 mo. Between-arm differences wereassessed with the Wilcoxon test.
Results: 1918 patients were randomized and 1428 patientscompleted the baseline QOL assessment. Compliance with QOL measures was over85%. Baseline summary scores for SF-36 physical (PCS, 47.5 for letrozole and47.9 for placebo) and mental (MCS, 55.5 for letrozole and 54.8 for placebo)were close to population norms (50). No differences were seen between groups inmean change scores for the SF-36 PCS and MCS and the other 8 QOL domains.Patients randomized to letrozole reported worse vasomotor symptoms (12 mo p =0.02, 36 mo p = 0.03) and worse sexual functioning (12 mo p = 0.01, 36 mo p =0.01). Further analyses with follow up to 60 months will be presented.
Conclusions: No differences were seen in overall QOLmeasured by the SF 36 summary measures between letrozole and placebo groups.Differences existed for vasomotor symptoms and sexual functioning which wereworse in the letrozole group and attributed to improvements in mean scores seenfor women randomized to placebo. The data indicate that continuation of AItherapy after 5 years prior treatment is not associated with a deterioration ofoverall QOL. Treatment related vasomotor and sexual functioning alterationsoccur with ongoing treatment. Clinical trial information:NCT00754845
打开微信 →→ 添加“医脉通肿瘤科”公众号,或扫描电脑屏幕右上方二维码 →→ 关注医脉通肿瘤科。随时随地获取肿瘤前沿资讯,一次打包最实用的肿瘤治疗知识。做科研达人、临床高手,尽在医脉通肿瘤频道。
