医脉通编译整理,转载请务必注明出处。
2016 ESMO大会上,乳腺癌内分泌治疗领域引人瞩目的FALCON研究结果公布,新型药物氟维司群使激素受体阳性晚期乳腺癌患者的无进展生存期得到显著延长,这种生存获益在疾病侵袭性较低,肿瘤较小的患者中尤为明显。
氟维司群有别于他莫昔芬(tamoxifen,TAM)及芳香化酶抑制剂(aromatase inhibitors,AIs),是具有新颖作用机制的内分泌治疗药物,它可以高亲和力地结合、阻断并降解雌激素受体(ER),既是雌激素受体的拮抗药同样也是下调药,但不会干扰自身雌激素水平。
FALCON是一项随机,双盲,多中心Ⅲ期临床试验,共纳入462例无法手术的局部晚期或转移性乳腺癌女性,患者均为ER阳性,HER阴性,此前未接受过其他激素治疗。随机将患者分配进氟维司群治疗(500mg,n=230)或阿那曲唑(1mg,n=232),两组患者均允许接受一线化疗。
中位随访25个月后结果显示,相较于阿那曲唑组,氟维司群组患者的PFS显著改善21%(16.6 vs 13.8个月)。进一步亚组分析显示,对于基线时肿瘤尚未侵犯肝脏或肺的乳腺癌患者,氟维司群对PFS的影响更为明显(22.3 vs 13.8个月)。
主要研究者,来自美国休斯敦贝勒医学院的Matthew Ellis博士表示:在无内脏侵犯的情况下,乳腺癌对生命的威胁并不十分紧迫。对于此类患者通常将内分泌治疗作为首选,目前来看氟维司群或许能成为新的标准治疗。
研究中,两干预组患者健康相关生活质量接近,而氟维司群和阿那曲唑最常见的不良反应分别为关节疼痛(16.7% vs 10.3%)和潮热(11.4% vs 10.3%)。Ellis博士认为,氟维司群与阿那曲唑的耐受性相似,但强于其他药物,未来在化疗或CDK4抑制剂联合应用中具有一定潜力。对于像老年人或肿瘤负荷低的患者,氟维司群是一个不错的低毒选择。
研究还发现,与阿那曲唑组相比,氟维司群治疗的持续缓解时间明显延长。这可能是氟维司群能够延长无进展生存期的原因之一。英国伦敦皇家马斯登内科肿瘤专家Nicholas Turner博士在评论该研究时表示,FALCON Ⅲ期研究结果反应了ER阳性,HER2阴性乳腺癌治疗的重要进展,提示早期使用氟维司群可能给患者提供更多的获益。
然而,在将该研究结果应用于临床实践前,需要注意两个问题:第一,FALCON仅纳入了未曾接受过内分泌治疗的乳腺癌,但临床中很多晚期患者在发病初期已经接受了治疗。其次,由于晚期乳腺癌治疗的不断进步,美国已批准CDK4/6抑制剂Palbociclib上市并可与AI联合使用,而FALCON研究设计是单药方案。未来还需要开展相关研究来帮助确定晚期乳腺癌的最佳用药顺序。
信源:Targeting Estrogen Receptor Improves Progression-free Survival in Advanced Breast Cancer.ESMO 2016 Press Release.
摘要原文
Abstract:LBA14
Title:FALCON: A phase III randomised trialof fulvestrant 500 mg vs. anastrozole for hormonereceptor-positive advanced breast cancer
Background: This Phase III,randomised, double-blind, multicentre trial (FALCON; NCT01602380) compared theselective estrogen receptor (ER) degrader (SERD) fulvestrant with anastrozolein patients with ER- and/or progesterone receptor-positive locally advanced ormetastatic breast cancer who had not received prior hormonal therapy.
Methods: Patients were randomised 1:1to fulvestrant (500 mg IM on Days 0, 14, 28, then each 28 days) or anastrozole(1 mg daily). The primary endpoint was progression-free survival (PFS),assessed via RECIST 1.1, surgery/radiotherapy for disease worsening, or death.Secondary endpoints were: overall survival (OS); objective response rate (ORR,complete response [CR] or partial response [PR]); duration of response (DoR);expected DoR (EDoR); clinical benefit rate (CBR; CR, PR, or stable disease ≥24weeks); duration of clinical benefit (DoCB); expected DoCB (EDoCB);health-related quality of life (HRQoL); and safety.
Results: In total, 462 patients(fulvestrant, n=230; anastrozole, n=232) were randomised. The primary endpointwas met as shown by a statistically significant improvement in PFS withfulvestrant vs. anastrozole (hazard ratio 0.797 [95% confidence interval 0.637,0.999]; p=0.0486; median PFS, 16.6 vs. 13.8 months, respectively). Secondaryoutcomes are shown in the Table (OS maturity was 31% at a median follow-up of25.0 months). Treatment impact on HRQoL was similar in both treatment groups.The most common adverse events were arthralgia (16.7% vs. 10.3%) and hot flushes(11.4% vs. 10.3%) for fulvestrant and anastrozole, respectively.
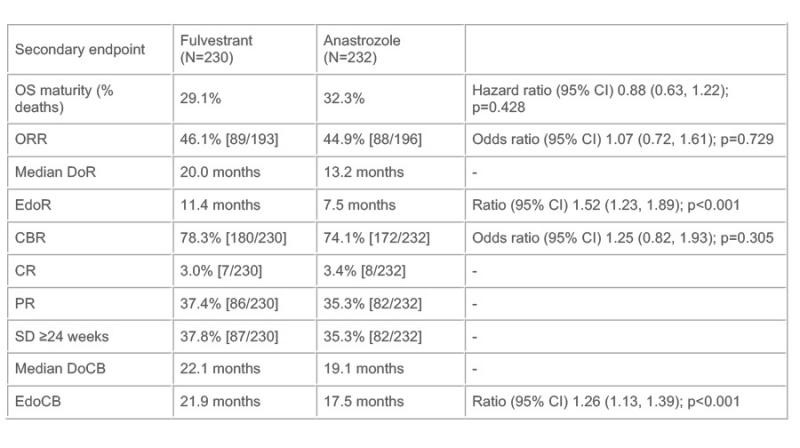
Conclusions: These results confirm the superior efficacy of fulvestrant over anastrozole in postmenopausal women with hormone receptor-positive locally advanced or metastatic breast cancer who have not received prior hormonal therapy.
