医脉通编译整理,转载请务必注明出处。
2016 ESMO大会上,除了闪耀全场的免疫治疗外,针对间变性淋巴瘤激酶(ALK)的靶向治疗研究也引人瞩目。Ⅲ期临床试验ASCEND-5结果显示,对于克唑替尼治疗后病情恶化的ALK阳性非小细胞肺癌(NSCLC),色瑞替尼与化疗相比可显著延长患者无进展生存期。
主要研究者,来自意大利都灵大学的Giorgio Scagliotti教授说:“ALK阳性NSCLC患者一线应首选ALK抑制剂克唑替尼,但绝大多数患者对克唑替尼耐药后,二线方案采用化疗。ASCEND-5是首个在克唑替尼治疗失败后,比较二代ALK抑制剂色瑞替尼是否优于化疗的Ⅲ期临床研究。”
该研究纳入231例NSCLC患者,随机进入色瑞替尼治疗或化疗(培美曲塞或多西他赛)。化疗组患者如出现疾病进展可交叉进入色瑞替尼组继续治疗。主要终点是无进展生存(PFS)。
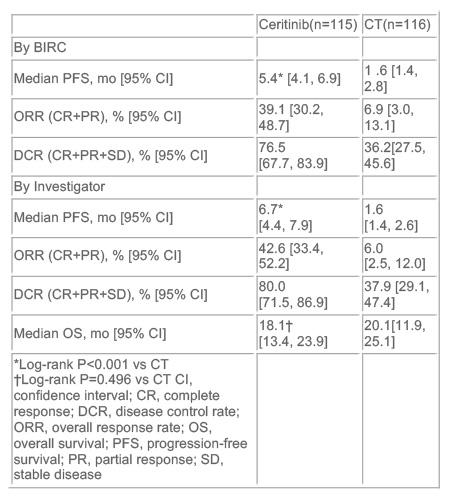
中位PFS在色瑞替尼治疗组为5.4个月,而化疗组仅有1.6个月(HR=0.49)。与化疗相比,色瑞替尼使总缓解率显著增加(39.1% vs 6.9%),但两干预组的总生存期并无明显差异。
化疗无效疾病进展,最后交叉进入色瑞替尼组治疗的患者共有75例。
Scagliotti教授表示,与化疗相比,NSCLC患者的PFS在色瑞替尼治疗下有明显延长。但并没有观察到总生存有多少提高,可能使因为交叉到色瑞替尼治疗的患者稀释了该药潜在的临床获益。
在毒性方面,该研究与之前的Ⅰ/Ⅱ期试验结果近似。最常见的3/4不良事件在色瑞替尼组是恶心(7.8%)、呕吐(7.8%)、腹泻(4.3%),在化疗组是中性粒细胞减少(15.5%)、乏力(4.4%)和恶心(1.8%)。与安慰剂相比,色瑞替尼显著提高了患者报告结局,包括肺癌特异性症状和总体健康状态。
“这项研究为克唑替尼治疗失败后的NSCLC创立了新的治疗策略。现在有理由确定给药顺序了,即先以克唑替尼为一线,耐药后过渡到色瑞替尼二线。” Scagliotti教授说道。
美国麻省总医院胸部肿瘤科主任Alice Shaw教授在评论该研究时表示:“这是第一个在ALK阳性患者二线治疗中比较二代ALK抑制剂和化疗的研究。先前的单臂试验提示,色瑞替尼和alectinib均可成为克唑替尼治疗失败后的二线治疗选择,而该Ⅲ期研究进一步证实了二代ALK抑制剂在提升PFS方面优于化疗。这将建立转移性ALK阳性肺癌克唑替尼转向色瑞替尼的治疗顺序。”
Shaw教授进一步总结道:“我们现在很期待二代ALK抑制剂在一线治疗中的试验结果,包括色瑞替尼 vs 化疗和alectinib vs 克唑替尼。后者将解决ALK阳性肺癌治疗领域最重要的问题,即谁才是ALK抑制剂的首选。”
信源:Ceritinib Provides Longer Progression-free Survival Than Chemotherapy in Phase III Trial of ALK Rearranged Lung Cancer Treatment. ESMO 2016 Press Release.
摘要原文
Abstract:LBA42
Title:Ceritinib vs chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with CT and crizotinib (CRZ): results from the confirmatory phase 3 ASCEND-5 study
Background: The ALK inhibitor CRZ is effective in ALK+ NSCLC pts, but most develop resistance and progressive disease (PD). Ceritinib showed robust efficacy in CRZ-pretreated pts in phase 1 & 2 studies. This multicenter, open-label phase 3 study (NCT01828112) compared efficacy of ceritinib vs CT in ALK+ NSCLC pts pretreated with CT & CRZ.
Methods: ALK+ NSCLC pts pretreated with 1–2 CT regimens & CRZ were randomized to oral ceritinib 750 mg/d or CT (pemetrexed [PEM] 500 mg/m2 or docetaxel [DOC] 75 mg/m2), stratified by WHO PS (0 vs 1–2) & brain metastasis at screening. Pts discontinuing CT due to PD could crossover to ceritinib. Primary endpoint was progression-free survival (PFS), assessed by blinded independent review committee (BIRC) per RECIST v1.1.
Results: Of 231 pts (median age 54 y), 115 were randomized to ceritinib and 116 to CT (PEM, n=40; DOC, n=73; 3 not treated). Median treatment exposure was 30.3 wk for ceritinib and 6.3 wk for CT. Median follow-up duration was 16.5 mo. Ceritinib showed statistically significant improvement in PFS (BIRC: median 5.4 vs 1.6 mo, HR=0.49, P<0.001) and increased overall response rate [95% CI] (BIRC: 39.1% [30.2, 48.7] vs 6.9% [3.0, 13.1]) vs CT (Table). Of pts discontinuing CT due to PD, 75 crossed over to ceritinib. Most frequent AEs (% any grade [% grade 3/4]) were diarrhoea (72.2 [4.3]), nausea (66.1 [7.8]) & vomiting (52.2 [7.8]) with ceritinib; fatigue (28.3 [4.4]), nausea (23.0 [1.8]), alopecia (21.2 [0]) & neutropenia (20.4 [15.0]) with CT. 6 (5.2%) ceritinib- and 8 (6.9%) CT-treated pts discontinued due to AEs. Ceritinib significantly improved lung cancer-specific symptoms (LCSS & QLQ-LC13) & overall health status (EQ-5D) vs CT (P<0.05).
Conclusions: Ceritinib showed a clinically meaningful and statistically significant improvement in PFS vs CT in pretreated ALK+ NSCLC.
